B-1.1 Heat and Heat Transfer
Heat is a form of energy. In North America, heating is commonly discussed using imperial units. Heat can be measured by its quantity and intensity. Before any heating system can be designed, it is necessary to understand what heat is, how it is transferred, and how that heat movement can be measured.
Quantifying Heat Energy
Heat quantity is described in British thermal units (BTU). One BTU is defined as the amount of energy it takes to raise one pound of water by one degree Fahrenheit. This is the approximate amount of energy released by burning one wooden match.
The metric unit of energy is the joule (J) or kilojoules (kJ). 1 BTU equals 1.054 kJ. The metric equivalent of the BTU is the kilowatt (kW). Note that the kilowatt incorporates one hour of time within its definition. Sometimes kilowatts are described as kilowatt hours.
Power is the rate at which energy is generated, converted, or used. The power ratings of heating and cooling equipment are expressed in BTU’s per hour (BTU/h) or kilowatts (kW). One kilowatt is equivalent to 3,412 BTU/h.
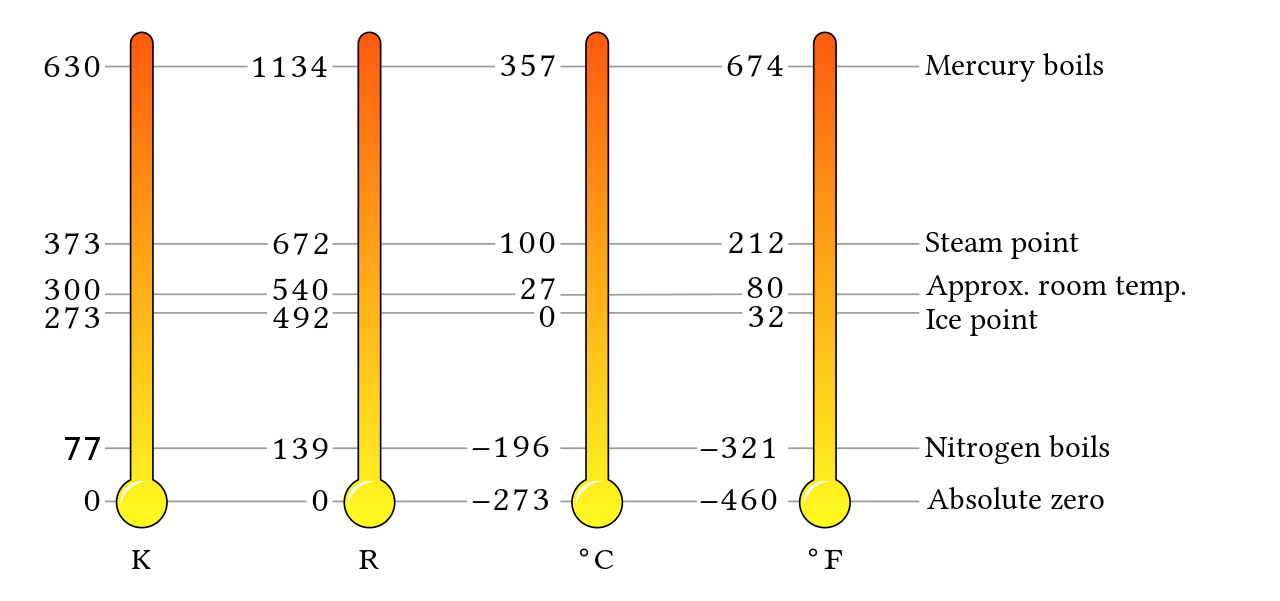
Temperature is the measure of heat intensity and is expressed in degrees Fahrenheit (°F) or degrees Celsius (°C). Fahrenheit and Celsius are often associated with imperial and metric scales, but that is not the case. Rather, they are parts of scales that have their baseline at the point where all heat is absent. This point is known as absolute zero.
The scale that shares degrees with the Fahrenheit scale is the Rankine scale. It has 460 of its degrees below 0°F — so 32°F would be expressed as 492°R (Rankine):
[latex]\color{blue}{32^\circ\text{F}+460=492^\circ\text{R}}[/latex]
The other scale that corresponds to degrees Celsius is the Kelvin scale. There are 273 degrees Kelvin between 0°C and absolute zero — so 100°C would be expressed as 373°K (Kelvin):
[latex]\color{blue}{100^\circ\text{C}+273=373^\circ\text{K}}[/latex]
Figure 1 compares the various temperature scales.
All matter is composed of atoms. All atoms vibrate, and this vibration is caused by the presence of heat. Even matter that is at temperatures below 0°C will vibrate because they contain heat. At absolute zero, all vibration stops, and no heat is present. The higher the intensity of atomic vibrations within a material, the greater the temperature of the material and the greater its heat content. Any material above absolute zero contains some measurable amount of heat energy. So, you could say that adding 10 BTU (a unit of heat) to a pound of water will increase the water’s temperature by 10°F (which measures the intensity of heat). Heat’s intensity is measurable with a thermometer, while the heat content of a material is the result of applying its temperature to a formula. A material’s heat intensity is a result of its heat content.
Heat Transfer
Heat always moves from an area of increased atomic activity (higher temperature) to an area of decreased atomic activity (lower temperature). Heat is transferred from one material to another by three main processes:
- Conduction
- Convection
- Thermal radiation
In a hot water heating system, heat transfer units or emitters use the processes of conduction, convection, and radiation to transfer heat to rooms or zones. The rate of heat transfer, known as heat flow, depends on the temperature difference between materials. Heat energy will continue to transfer from warm material to cool material until both are the same temperature.
Conduction
Conduction is the transfer of heat by contact. Molecules vibrate in response to their level of heat energy. When two molecules bump into each other, the vibration of one affects the other, and heat energy is transferred. When materials that have different temperatures are in contact, heat from the warm material is conducted to the cool material (Figure 2).
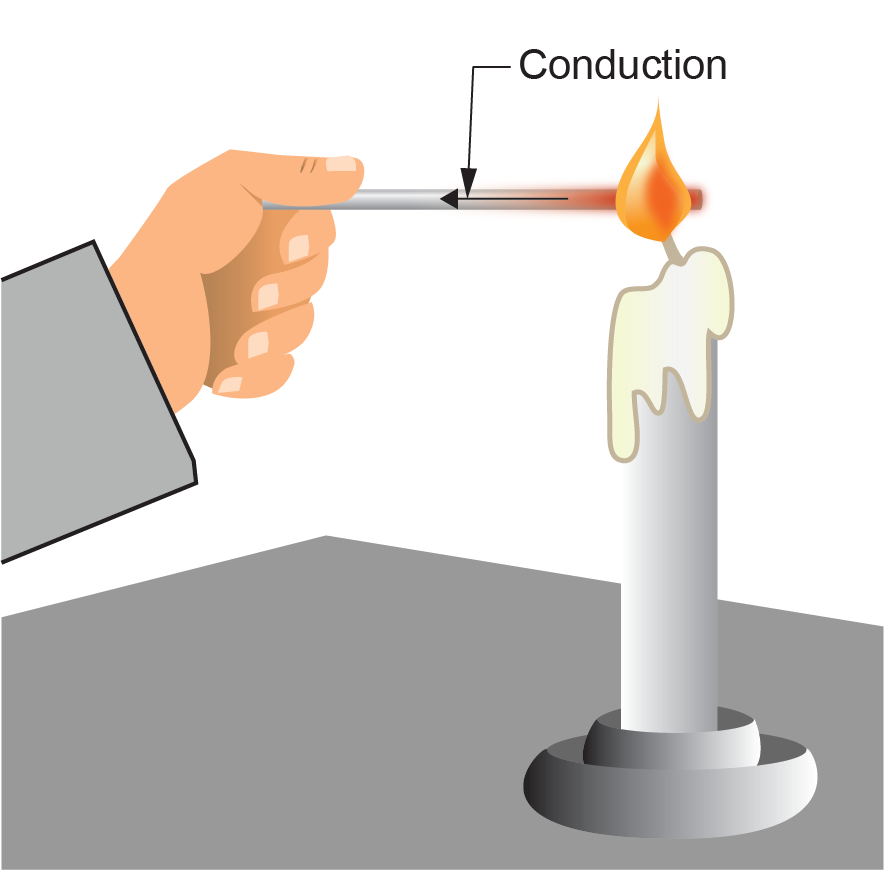
Effects of Conduction
Hot water loses heat through the walls of containing vessels and piping. Water in piping has molecular contact with the piping walls. This contact causes heat to conduct through the piping walls to outside air.
The speed at which heat energy transfers through conduction depends on the difference in temperature between the two materials. If the temperature difference is doubled, the heat flow rate also doubles.
The amount of heat transferred is also affected by the amount of surface area in contact. If the surface area in contact is doubled, the amount of heat transferred also doubles.
All materials allow some movement of heat. Some materials, such as metals, allow heat to conduct very rapidly. Other materials, like air, allow heat to conduct slowly. Heat conductivity (or thermal conductivity) is the speed at which a material is able to transfer heat. A material with low heat conductivity is called an insulator. The lower the heat conductivity, the greater the insulation value. Table 1 lists the heat conductivity of several common substances with a thickness of 1 in., a surface area of 1 sqft, and a temperature difference across the material of 1°F.
| Substance | Conductivity |
|---|---|
| Copper | 2660 BTU/h |
| Steel | 320 BTU/h |
| Concrete | 12 BTU/h |
| Water | 4.1 BTU/h |
| Plastic | 1.2 BTU/h |
| Air | 0.15 BTU/h |
Copper conducts heat more quickly than steel, and steel conducts heat more quickly than plastic. Plastic conducts so little heat that it is classed as an insulator. Plastic’s low conductivity and high corrosion resistance make it an ideal material for moving water within a heating or cooling system.
Convection
Convection is the transfer of heat in a fluid (a gas or a liquid) caused by a difference in densities. It is sometimes called gravity circulation. When heat is applied to a fluid, the temperature of the fluid increases, causing it to expand and become less dense than the surrounding fluid. It is then pushed upward as cooler, denser fluid flows downward to take its place. This upward push by cooler, denser fluid is known as buoyancy. Early hydronic heating systems depended on gravity circulation to move heat from the heat source (boiler) through piping to the heat transfer units (cast-iron radiators) in the heated areas.
Air in contact with the radiators was heated by conduction, which in turn created movement of heated air in the room through convection (Figure 3). This process of heating the air is still used by modern convectors, although the circulation of water is accomplished through the use of pumps.
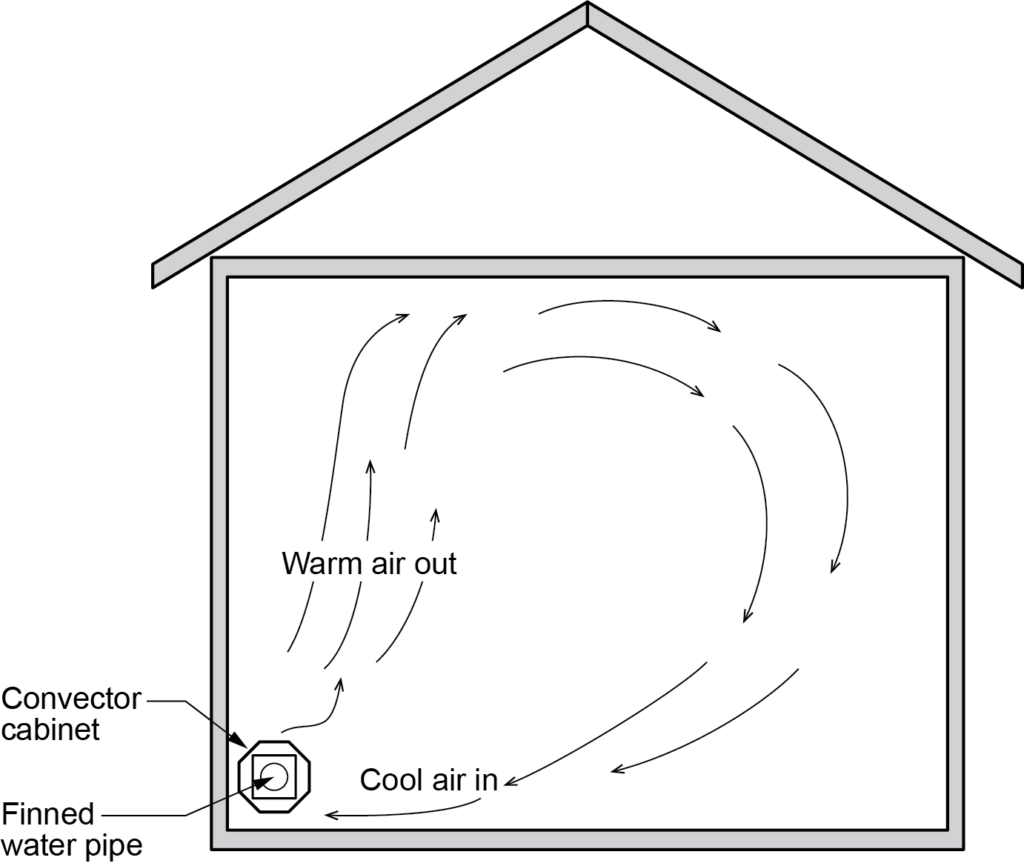
Radiation
Radiation is the transfer of heat by electromagnetic heat rays emitted directly from a heat source to a receiving material. Heat rays travel through space in the same way that light rays travel. It is important to note that radiant heat does not raise the temperature of the air between the source and the material. Heat rays are rarely impeded or absorbed by intervening air due to air’s lack of mass or density. When the heat rays reach a material other than air, that material absorbs them and becomes warmer.
A good example of a radiant heat source is the sun (Figure 4). Imagine you are outside on a very cold yet sunny day. If you stand facing the sun, you will feel warmth on your face, but not on your back. On the other hand, if you turn away from the sun, your back warms up, but your face will feel cold. The sun’s rays are heating you directly, rather than heating the air between it and you.
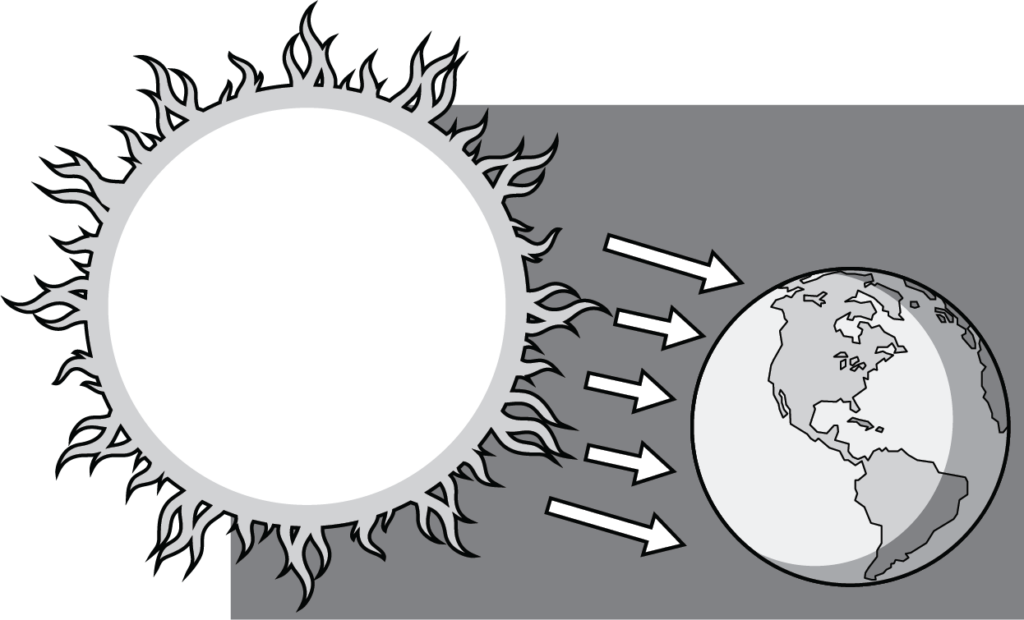
Radiant Heat
The amount of heat transmitted by a radiator is proportional to its surface area and temperature. The best radiators of heat are rough and black, such as cast-iron stoves. Light-coloured, smooth, and shiny materials, such as toasters and kettles, tend to reflect heat. As the temperature of the radiating material increases, the amount of radiation increases.
 Self-Test B-1.1: Heat and Heat Transfer
Self-Test B-1.1: Heat and Heat Transfer
Complete Self-Test B-1.1 and check your answers.
If you are using a printed copy, please find Self-Test B-1.1 and Answer Key at the end of this section. If you prefer, you can scan the QR code with your digital device to go directly to the interactive Self-Test.

References
Baltakatei Sandoval, S. (2023, February 8). Temperature scales comparison (K,R,C,F) [digital image]. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Temperature_scales_comparison_(K,R,C,F).svg&oldid=730895952 (last revision). (File derived from: Temperature-scales-comparison.svg by MikeRun)
Skilled Trades BC. (2021). Book 1: Fuel gas systems, heating and cooling systems. Plumber apprenticeship program level 2 book 1 (Harmonized). Crown Publications: King’s Printer for British Columbia.
Trades Training BC. (2021). B-1: Describe types of heating and cooling systems. In: Plumber Apprenticeship Program: Level 2. Industry Training Authority, BC.
Media Attributions
All figures are used with permission from Skilled Trades BC (2021) unless otherwise noted.
- Figure 1 Comparison of the various temperature scales is by Stephen Baltakatei Sandoval (2023) via Wikimedia and is used under a CC BY-SA 4.0 licence.
Long Description: Figure 1 Comparison of the Various Temperature Scales
The following table shows a comparison of the various temperature scales: Rankine, Fahrenheit, Celsius, Kelvin.
| Rankine (°R) | Fahrenheit (°F) | Description | Celsius (°C) | Kelvin (°K) |
| 1134 | 674 | Mercury boils | 357 | 630 |
| 672 | 212 | Water boils; steam point | 100 | 373 |
| 492 | 32 | Water freezes | 0 | 273 |
| 460 | 0 | — | -18 | 256 |
| 139 | -321 | Nitrogen boils | -196 | 77 |
| 0 | -460 | Absolute zero | -273 | 0 |
Back to Figure 1

